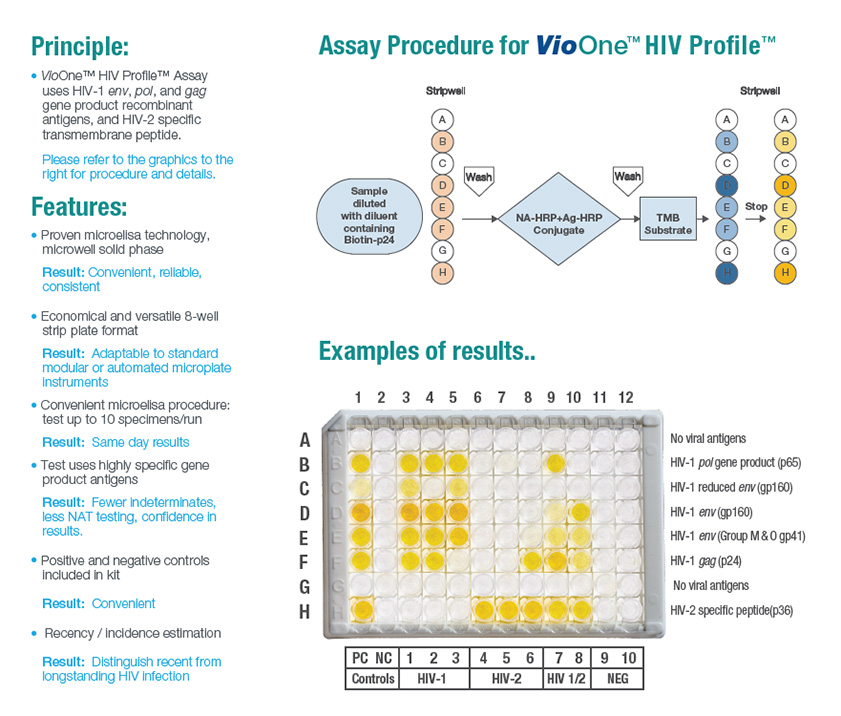
VioOne™ HIV Profile™
Supplemental Assay
Serologic assay for detection and differentiation of antibodies
directed to different gene products of HIV-1 and HIV-2
HIV Profile Intended Use
For in vitro Diagnostic Use
The VioOne™ HIV Profile™ Supplemental Assay is an enzyme-linked immunosorbent assay
(ELISA) for confirmation and differentiation of individual antibodies directed to various gene
products of HIV-1 (Group M & Group O) and HIV-2 in human serum or plasma. It is a more
specific test used to confirm the presence of HIV-1 and/or HIV-2 antibodies from specimens
that were repeatedly reactive in diagnostic screening procedures.