VioOne™ HIV Profile™ Supplemental Assay
For In Vitro Diagnostic Use
Serologic assay for detection and differentiation of antibodies directed to different gene products of HIV-1 and HIV-2
The VioOne™ HIV Profile™ Supplemental Assay is an enzyme-linked immunosorbent assay (ELISA) for confirmation and differentiation of individual antibodies directed to various gene products of HIV-1 (Group M & Group O) and HIV-2 in human serum or plasma. It is a more specific test used to confirm the presence of HIV-1 and/or HIV-2 antibodies from specimens that were repeatedly reactive in diagnostic screening procedures.
Format
Economical and versatile 8-well strip plate format that is adaptable to standard modular or automated microplate instruments
Antigens
Tests use highly specific gene product antigens resulting in fewer indeterminates, less NAT testing, and true confidence in results
Procedure
Convenient microelisa procedure to test up to 10 specimens per run with same day results
Principle
Materials Provided
Requirements
Results
Principle
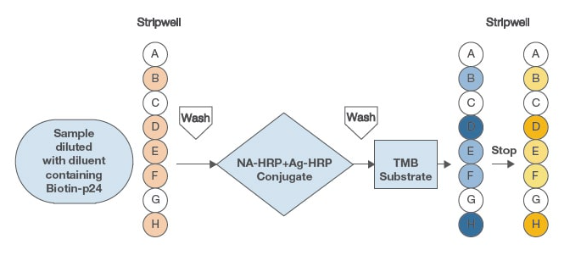 VioOne™ HIV Profile™ Assay uses HIV-1 env, pol, and gag gene product recombinant antigens, and HIV-2 specific transmembrane peptide. See above for procedure and details.
VioOne™ HIV Profile™ Assay uses HIV-1 env, pol, and gag gene product recombinant antigens, and HIV-2 specific transmembrane peptide. See above for procedure and details.
Materials Provided
VioOne™ HIV Profile™ Strips
Sample Diluent
Negative Control Serum (Human)
HIV-1/2 Positive Control Serum
Conjugate
Conjugate Diluent
TMB Solution
Peroxide Solution
Wash Buffer Concentrate (provided separately as an accessory)
Plate sealers- adhesive
Requirements
Note: For any instrument, the manual provided by the manufacturer should be reviewed for additional
information regarding the following:
1. Installation and special requirements.
2. Operation principles, instructions, precautions, and hazards.
3. Equipment calibration.
4. Manufacturer’s specifications and performance capabilities.
5. Service and maintenance information.
6. Quality Control.
Automated diluter/dispenser system (minimum 10 µl with 10% accuracy), test tubes, or equivalent
Aspiration/wash system: The aspiration/wash system must be capable of dispensing a minimum volume of 300 µl, and capable of performing a minimum 30 second soak cycle. Aspirated waste must be contained in a closed system.
Adjustable multi-channel variable volume pipet system capable of delivering 50 – 300 µl ± 5%, and tips.
Adjustable multi-channel variable volume pipet system capable of delivering 5 – 50 µl ± 5%, and tips.
Micropipet(s) capable of delivering, 10 µl ± 10%, 1000 µl ± 5%, and tips
Incubator: A dry incubator or equivalent, capable of maintaining 37 ± 2°C.
Microplate reader: Any microplate reader capable of transmitting light at 450 nm ± 5 nm with a linear absorbance range
of 0 to 2.000.
Timer
Graduated cylinder, 50 ml and 1-2.5 L or equivalent
Reagents/Disposables
2N Sulfuric Acid
Purified Water, USP7 or NCCLS Type I
7 reagent water, or equivalent
Stripholder with uncoated wells
Absorbent paper
V-shaped disposable troughs or equivalent
Disposable gloves
Sodium hypochlorite solution (5%) or liquid bleach
Appropriate biohazard waste containers for materials potentially contaminated with infectious agents
Results
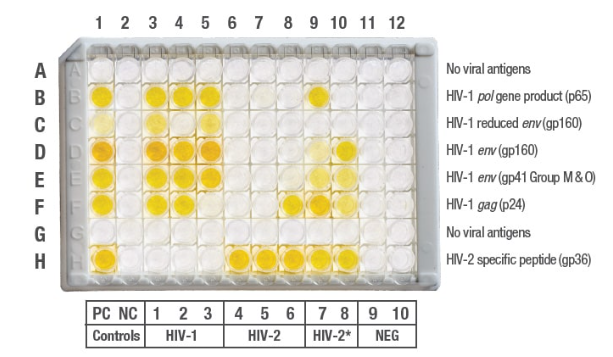
*HIV-2 positive with reactivity to HIV-1 antigens. Refer to package insert.
